On April 15, Hengrui Pharmaceutical announced its 2017 annual report with an operating income of 13.836 billion yuan (+24.72%). The net profit attributable to shareholders of listed companies was 3.217 billion yuan (+24.25%), and the non-net profit was 3.101 billion. Yuan (+19.76%).
The market value of Hengrui Pharmaceutical has stood at 250 billion yuan. Although the pharmaceutical sector fell in the previous two days, Hengrui Pharmaceuticals fell 4.65% on the day and fell to a market value of 10 billion. However, the current market value is still close to 220 billion yuan.
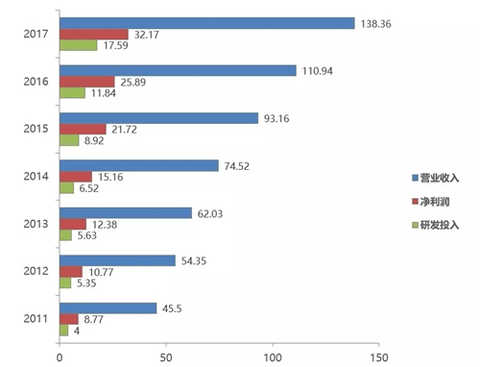
Hengrui Pharmaceutical's revenue, net profit and R&D investment in the past 7 years (unit: 100 million yuan)
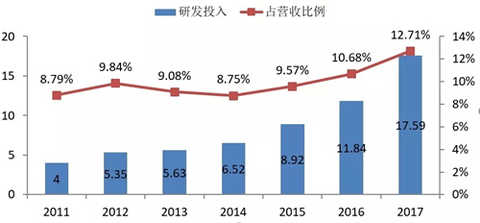
Hengrui Medicine's R&D investment growth trend in the past 7 years
Hengrui's economic indicators have grown steadily in 2017. The main drivers are the following three aspects:
The first is the harvest of innovation. The gradual gains of innovations have played a role in driving the growth of Hengrui's performance.
The second is the income from the export of preparations. Hengrui's export preparation products have grown steadily in the foreign regulated market, which has promoted Hengrui's operating income and profit growth.
The third is the optimization of Hengrui product structure. With the adjustment of Hengrui's product structure, the situation of a single anti-tumor drug has been gradually changed over the years. Hengrui anti-tumor drugs, represented by surgical anesthesia, contrast agents and characteristic infusions, gradually expand the market in their respective therapeutic fields and continue to maintain steady growth.
In the future, Hengrui will continue to steadily promote the internationalization of R&D innovation and formulation products. At the same time, it will also focus on the optimization and improvement of product structure to ensure the sustainable growth of Hengrui's performance.
Business revenue
Hengrui Pharmaceutical's main business involves drug research and development, production and sales. Its main products cover anti-tumor drugs, surgical anesthesia drugs, special infusions, contrast agents, cardiovascular drugs and many other fields.
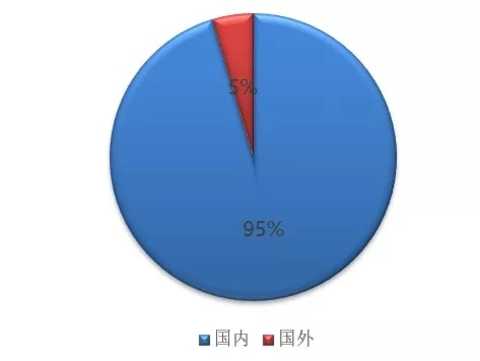
In Hengrui's revenue of 13.836 billion yuan in 2017, domestic revenue accounted for 13.187 billion (95.31%), foreign accounted for 637 million (4.69%), and overseas income increased by 0.8 percentage points from 2016.
Hengrui's revenue growth is mainly from oncology drugs, contrast agents and surgical anesthesia products. The rapid release of innovative drugs such as apatinib and oncology drugs still maintain a leading position in the market. Among them, the innovative drug apatinib entered the national medical insurance catalogue, and the market sales scale achieved another good result; the sales of contrast agent products increased by 44.85% compared with last year. It ranks first in the domestic market in this field; surgical anesthesia products increased by 19.71%.
Hengrui continues to steadily promote R&D innovation and internationalization of preparation products. At the same time, it is also focusing on the optimization of product structure, and its performance is expected to continue to maintain rapid growth.
R&D investment
The most eye-catching aspect of Hengrui's 2017 annual report is that R&D expenses continue to soar (+48.53%), which is higher than +32.82% in 2016. The research and development expenses in 2017 reached 1.759 billion yuan, which is far ahead of the pharmaceutical companies that have published annual reports (Basic China 270 million US dollars, China Biopharmaceutical 1.595 billion, Fosun Pharma 1.529 billion yuan), providing project development and innovation. Strong support.
Hengrui has built a research and development team of more than 2,000 people, including more than 1,000 doctors, masters and more than 100 foreign employees. It also has R&D centers in the United States, Japan and China, and insists on annual sales of about 10%. R&D funding. Hengrui's innovation model has gradually moved from the initial “me-too†and “me-better†to the source innovation, and the innovative drug layout is transforming from small molecule drugs to macromolecular drugs, resulting in an antibody toxin fusion with independent intellectual property rights (ADC). The technology platform has mastered the development technology of tumor immune antibody series products. In the field of biotechnology representing the development direction of the global pharmaceutical industry, the company has set up a series of research and development platforms, and is the first to apply for international leading antibody toxin conjugates in China. ADC drug (biological missile). Up to now, the company's two innovative drugs, Ericsib and Apatini, are listed. In 2017, SHR-A1403, SHR-9146, SHR-1316 injection, SHR-8554 injection, SHR-1314 injection and other products for injection were approved for clinical trials overseas.
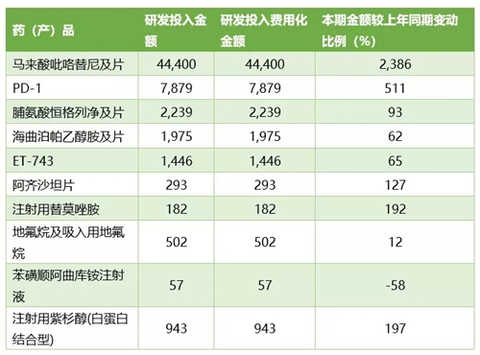
Basic information of major R&D projects in 2017 (unit: 10,000 yuan)
Formulation internationalization
In 2017, Hengrui continued to strengthen the implementation of its internationalization strategy, actively expand overseas markets, and achieved overseas sales income of 637 million yuan. In the internationalization of generic drugs, fensulfame sulbactam injection, docetaxel injection, dexmedetomidine hydrochloride injection were approved in the United States, and caspofungin and desflurane for injection were approved in Europe. The sales of series products such as cyclophosphamide for injection increased steadily; the European, American and Japanese high-end regulatory market projects were registered as planned, and in 2017, 4 injections and 2 APIs were submitted to the US FDA for submission to Japan. 1 injection, submitting a registration application for 1 tablet and 1 drug substance to Europe; other emerging markets such as Australia, South Africa, Middle East and other countries are gradually strengthening registration. For the internationalization of innovative drugs, SHR-A1403, SHR9146, SHR-1316 injection, SHR8554 injection, SHR-1314 injection and other products for injection have been approved for clinical trials overseas.
2018 development plan
In 2018, Hengrui will continue to adhere to the two strategies of “scientific and technological innovation†and “internationalizationâ€, enhance crisis awareness, integrate superior resources, improve work efficiency, comprehensively deploy China Hengrui, and strive to build the world Hengrui. Focus on the following aspects:
In terms of sales, Hengrui will be market-oriented, customer-oriented, rationally allocate resources, further expand sales network, strengthen specialization and brand sales, and strive to do the following work: First, aim at the current new situation, with professionalization Teams, systems, and norms to solve access problems; second, strengthen regional management, actively, rationally, and effectively use local resources to truly build China Hengrui's network structure; third, promote sales specialization, strengthen personnel training and management To improve performance with team operations.
In terms of R&D, Hengrui will continue to improve R&D efficiency, focus on the quality and timeliness of R&D, and focus on innovative drugs, generic drugs, overseas clinical and consistency evaluations, and earnestly implement R&D registration and reporting programs. By establishing a professional and hard-working CRA team, we will accelerate clinical innovation research and promote the listing of Phase II and Phase III clinical varieties as soon as possible, so that R&D and sales will form a virtuous circle. In terms of specific work, the first is to have the spirit of reform and innovation, to dare to break through the inherent mode of thinking, to solve the inherent problems in the work with new ideas and new methods; the second is to strengthen implementation and assessment, and to use the project as a starting point to introduce a competitive mechanism. To ensure that all work can be promoted and implemented in place; third, reasonable and dialectical handling of compliance and efficiency issues, and to improve work efficiency on the basis of ensuring compliance. Through the "Scientific Plan + Process Management + Resource Support + Team Stability" and other measures to ensure the smooth implementation of the R&D program. In terms of internationalization, internationalization is the strategic direction that Hengrui has adhered to for many years, and it is also an important support for Hengrui to achieve leapfrog development in the future.
In 2018, Hengrui will adhere to the domestic and foreign markets and do a good job in the mutual response of the two markets. First, in the field of generic drugs, we will focus on the sales of Dahengrui drugs in Europe, America, Japan, Hong Kong, South America, etc., and strive to bring Hengrui's overseas sales to a new level. Second, in terms of innovative drugs, Hengrui needs one step. A footprint, solidly promote the overseas clinical practice of innovative drugs, and strive to sell Hengrui innovative drugs overseas as soon as possible.
In terms of production and quality, Hengrui will continue to implement the policy of “Compliance, Efficiency, Intelligence and Greenâ€. First, do a good job of decomposing the annual production plan in advance to ensure market supply, cooperate with R&D to do a good job in clinical preparation and listing verification of new varieties; second, rationally allocate resources and do a good job in staffing and equipment hardware requirements under the premise of compliance management. The dynamic management, especially the laboratory and process change work; the third is to strengthen the automation and intelligent construction, to develop three-year and five-year plans, to the level of Europe and the United States, and strive to achieve the level of Hengrui automation in Europe and the United States in the next 3-5 years The fourth is to strengthen supplier management, reduce exogenous complaints, strengthen employee training, and improve the quality awareness of all employees.
In terms of industrial layout, Hengrui will further open up and expand the industrial layout, establish and improve the four major operation centers, radiate the country, gather talents, and conform to the development of products and markets.
In terms of talent development, the first is to further improve the quality of the human resources department, improve the job and professional promotion channels, make the incentive mechanism more suitable for the development needs of Hengrui, and strengthen performance appraisal; second, emancipate the mind, Hengrui will follow the authority and ability The idea is to adhere to the combination of introduction and self-training, and to build a good team of talents; the third is to strengthen the training of cadres and enhance the ability of cadres to learn and accept new things.
Editor in charge: Chen Wei
This football training suit is not only specially designed for football, but also a very good sportswear.
Not only is it breathable and absorbing sweat, the material is also very soft, feels very good, and has a certain anti-slip effect, which ensures the performance during kicking and sports.
This football training uniform is suitable for all ages and figures. There are many sizes. If you have any needs and suggestions, please contact us.
Football Wear,Football Training Wear,Soccer Wear,Soccer Training Wear
Yangzhou Youju E-commerce Co.,Ltd , https://www.yzxygarment.com